Clomiphene Citrate Success: Role of Ethnicity and Genetics
Clomiphene citrate (Clomid) is a widely used medication to treat ovulatory infertility, particularly in women with PCOS. While it helps many women ovulate, success rates vary due to factors like genetics, ethnicity, metabolic health, and access to care. Here's what you need to know:
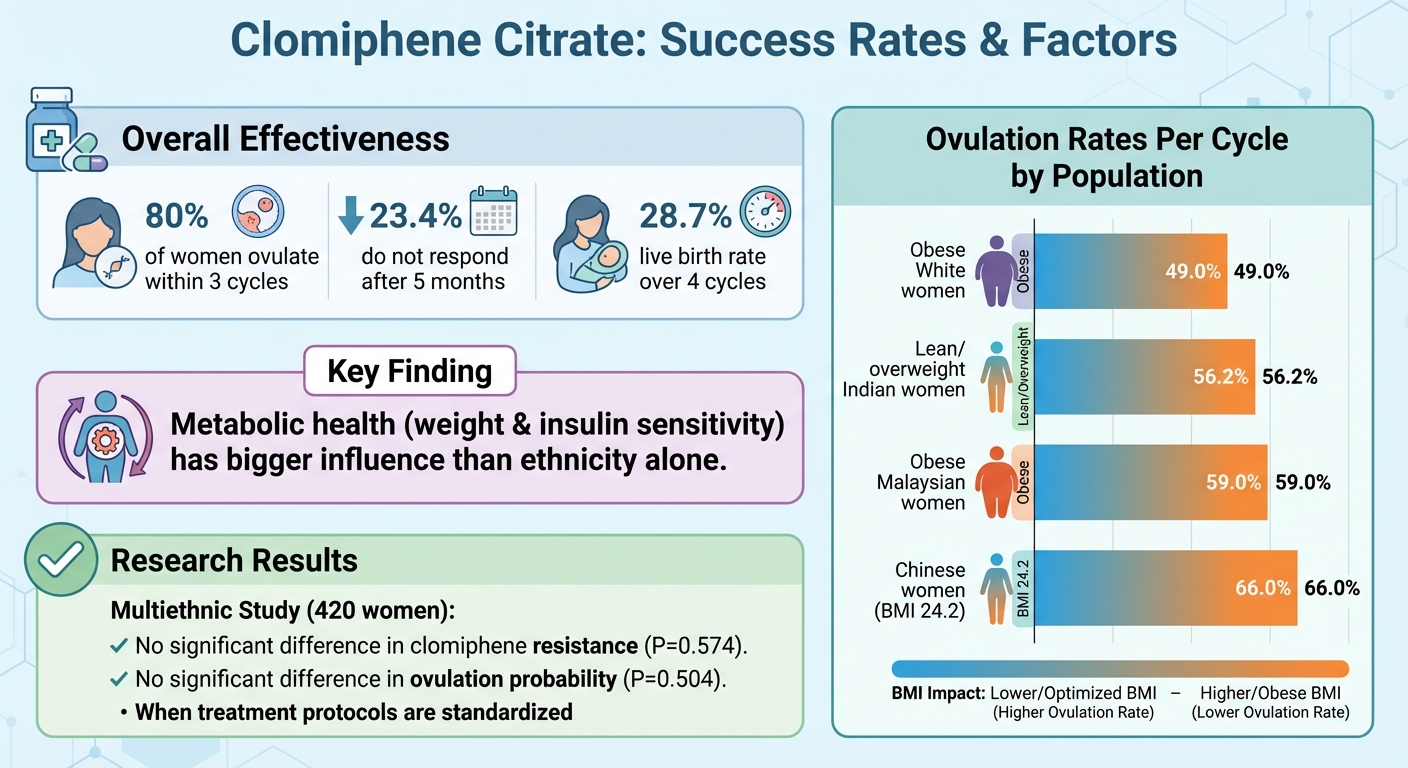
- Effectiveness: About 80% of women ovulate within three cycles, but 23.4% may not respond after five months. Live birth rates are 28.7% over four cycles.
- Ethnicity & Genetics: Genetic differences in hormone receptors and insulin signaling impact treatment response. Ethnicity influences metabolic factors like BMI and insulin resistance, which can affect ovulation rates.
- Access Disparities: Women from minority groups in the U.S. face delays in fertility care due to structural barriers like cost and insurance coverage.
- Improving Outcomes: Pre-treatment evaluations, weight management, and combining clomiphene with insulin-sensitizing treatments (e.g., metformin) can boost success. Telehealth services like Oana Health help reduce access gaps.
Personalized treatment, considering genetic and metabolic profiles, combined with better access to care, can improve clomiphene outcomes for diverse populations.
How Ethnicity Affects Clomiphene Citrate Results
Clomiphene Citrate Success Rates and Ovulation by Ethnicity and BMI
Ethnicity and socioeconomic factors can play a significant role in how individuals respond to clomiphene citrate, adding layers of complexity to fertility treatments.
PCOS Symptoms Vary by Ethnicity
Polycystic ovary syndrome (PCOS) doesn't look the same for everyone, and ethnic background can shape how it presents. A 2022 study involving 420 women with PCOS from Northern European, Mediterranean, African, South‑East Asian, and South American backgrounds highlighted some key differences. These included variations in BMI, waist circumference, LH levels, insulin levels, and androgen concentrations. For instance, women from non‑Northern European backgrounds often had higher BMIs, more central fat distribution, and greater insulin resistance, all of which are linked to lower ovulation rates when using clomiphene.
Here’s a snapshot of ovulation rates per cycle from the study:
- 49.0% in obese White women
- 56.2% in lean or overweight Indian women
- 59.0% in obese Malaysian women
- 66.0% in Chinese women with an average BMI of 24.2 kg/m²
This suggests that metabolic health - especially factors like weight and insulin sensitivity - has a bigger influence on clomiphene response than ethnicity alone. For example, South Asian and Middle Eastern women often struggle with insulin resistance and metabolic syndrome even at lower BMIs. Similarly, Black and Latina women in the U.S. frequently face higher rates of obesity, hypertension, and metabolic syndrome, which can negatively affect treatment success.
But biology isn’t the only factor. Socioeconomic challenges also play a major role in shaping outcomes.
Access Barriers and Socioeconomic Factors
Healthcare disparities can significantly impact treatment outcomes. In the U.S., Black, Hispanic, Native American, and some Asian American women often face delays in receiving fertility evaluations and treatments compared to White women - even when income and education levels are comparable. Gaps in insurance coverage and high out-of-pocket costs make it harder to access necessary monitoring and dose adjustments. In many states, infertility treatments aren’t covered by insurance, and even when they are, medications like clomiphene may not be included.
Other barriers include work schedules and transportation issues, especially for women in lower-wage jobs or those working multiple shifts. These challenges can make it tough to attend time-sensitive appointments, further delaying treatment and exacerbating metabolic issues.
Research Gaps and the Need for Better Data
A lack of diverse research complicates our understanding of how ethnicity and socioeconomic factors influence clomiphene outcomes. The 2022 multiethnic study was groundbreaking in its direct focus on ethnicity and clomiphene ovulation outcomes. It found no significant differences in clomiphene resistance (P=0.574) or predicted ovulation probability (P=0.504) when treatment protocols were standardized. However, most major studies have included few non‑White participants or have been conducted in relatively homogeneous populations.
For example, a large Chinese trial involving 1,000 women provided valuable insights for that population but didn’t address ethnic differences more broadly. Without larger, more diverse studies, it’s hard to determine whether subtle pharmacologic differences exist or if disparities are primarily driven by access and metabolic health. Researchers from the multiethnic study emphasized the need for bigger, more diverse cohorts to avoid overstating access-related disparities or overlooking important biological differences. Comprehensive data that considers both biological factors (like BMI and insulin resistance) and social determinants (such as income and access to care) is essential for tailoring treatments to individual needs.
Genetic Factors That Affect Clomiphene Citrate Response
Genetics play a key role in how effective clomiphene citrate is for inducing ovulation. Research has pinpointed several genes that can influence how well a person responds to treatment, often in combination with factors like body weight and insulin levels.
Genes That Influence Clomiphene Response
The response to clomiphene is tied to several genetic pathways, including estrogen receptor genes (ESR1 and ESR2), androgen pathway genes (CYP17A1, CYP19A1, AR), and insulin signaling genes (INSR, IRS-1, PPAR-γ). Variations in ESR1 and ESR2 may determine the dosage needed to trigger ovulation. While some women respond to the standard starting dose, others might require higher amounts or see little effect at all.
Similarly, changes in androgen pathway genes can result in higher androgen levels or alter the conversion of androgens into estrogen, which might contribute to clomiphene resistance. For women with genetic predispositions to insulin resistance, ovulating on clomiphene alone can be challenging. However, combining clomiphene with insulin-sensitizing treatments, like metformin or lifestyle adjustments, often improves outcomes.
How Genetic Variants Differ by Ethnicity
The prevalence of these genetic variations can differ significantly across ethnic groups in the United States. Research has shown that allele frequencies for genes like ESR1/2, AR, FSHR, and those involved in insulin signaling vary among non-Hispanic White, Black, Hispanic/Latina, and Asian populations. For instance, genetic variants linked to androgen excess and insulin resistance are more common in South Asian and Hispanic women, while some estrogen receptor variants are more frequently observed in individuals of East Asian or European ancestry.
Interestingly, these genetic differences don’t always translate into varied treatment outcomes. A 2022 study involving 420 women with PCOS from diverse ethnic backgrounds found no significant differences in clomiphene resistance (P = 0.574) or the likelihood of ovulation (P = 0.504) when a standardized treatment protocol was used. This suggests that while genetic profiles vary, standardized treatment approaches can yield similar ovulation success rates. Such findings highlight the importance of tailoring treatment to individual needs rather than focusing solely on genetic predispositions.
Why Genetic Testing Isn't Standard Yet
Despite these genetic insights, they haven’t yet reshaped clinical practice. Currently, no genetic test is available to guide clomiphene dosing because the evidence linking specific gene variants to treatment outcomes is still largely associative. Many studies have been small and lack replication, making it unclear whether genetic information can reliably improve pregnancy rates or shorten the time to conception.
Another barrier is cost. Genetic testing for clomiphene response is not typically covered by insurance, and clinical guidelines still prioritize established predictors like age, BMI, ovarian reserve, and PCOS characteristics when crafting treatment plans. Until larger, more diverse studies confirm that a genetics-based approach is both effective and affordable, genetic testing is unlikely to become a standard part of clomiphene treatment.
sbb-itb-6dba428
How to Improve Clomiphene Citrate Treatment Outcomes
Tailoring clomiphene citrate treatment to an individual's metabolic profile, body composition, and specific PCOS characteristics can significantly improve ovulation success. This personalized approach involves careful pre-treatment evaluations, metabolic health management, and thoughtful adjustments to treatment protocols.
Customized Pre-Treatment Evaluations
Before starting treatment, it’s essential to gather detailed information about a patient’s metabolic and hormonal status. This includes measuring BMI, waist circumference, blood pressure, fasting glucose, insulin levels, and lipid profiles to identify potential metabolic risks. Hormonal assessments - covering testosterone, DHEAS, LH, FSH, AMH, TSH, and prolactin - along with transvaginal ultrasounds to evaluate antral follicle count and ovarian morphology, help predict treatment outcomes and assess risks like multiple pregnancies.
PCOS phenotype plays a critical role in treatment planning. Women with hyperandrogenic PCOS and obesity require different strategies compared to lean women with normoandrogenic PCOS. Studies show that focusing on individual metabolic factors rather than ethnicity leads to more effective treatment protocols, ensuring consistent results with minimal clomiphene doses and reduced resistance rates.
Managing Weight and Metabolic Health
Body weight has a significant impact on ovulation rates. For instance, women with a BMI around 24.2 kg/m² achieve ovulation rates of about 66%, compared to 49–59% in those with obesity. Even modest weight loss - around 5–10% of body weight - can help restore ovulation and improve clomiphene’s effectiveness. For a woman weighing 220 pounds, losing just 11–22 pounds through a combination of calorie reduction and increased physical activity can be a manageable and impactful goal.
Insulin resistance also reduces ovarian responsiveness to clomiphene. Women with insulin resistance, impaired glucose tolerance, or metabolic syndrome may benefit from combining clomiphene with metformin. Clinical trials have shown that this combination significantly improves ovulation and pregnancy rates compared to either treatment alone.
For those who experience gastrointestinal side effects from oral metformin, topical formulations can provide a more tolerable alternative. Services like Oana Health offer prescription-based treatments for managing PCOS-related insulin resistance and weight issues. They provide convenient home delivery, which is especially helpful for patients in rural or underserved areas. Topical metformin starts at $89 per month, while extended-release oral metformin is available for $22 per month.
Adjusting Treatment Protocols for Better Results
Once pre-treatment factors are optimized, fine-tuning the treatment protocol can further enhance success rates. The standard starting dose is 50 mg daily for five days (typically beginning on cycle days 3–5). If ovulation doesn’t occur, the dose can be increased in 50 mg increments, up to a maximum of 150–200 mg. Ovulation can be confirmed by monitoring mid-luteal progesterone levels (about seven days after ovulation) or using transvaginal ultrasound to track follicle development. Ultrasound also helps with timing intercourse or intrauterine insemination (IUI).
Age and cycle number significantly influence success rates. For example, in a study of nearly 4,000 clomiphene-IUI cycles for unexplained infertility, pregnancy rates per cycle were 11.5% for women aged 35–37, 7.3% for ages 38–40, 4.3% for ages 41–42, and just 1.0% for those over 42. These figures emphasize the importance of setting realistic expectations and knowing when to consider alternative treatments.
Clomiphene is typically deemed unsuccessful if ovulation doesn’t occur at maximum doses after 3–6 cycles, or if pregnancy isn’t achieved despite ovulation and well-timed attempts. In a trial involving 1,000 Chinese women with PCOS, clomiphene achieved a live birth rate of 28.7%, compared to 15.4% with placebo. However, 23.4% of participants didn’t ovulate after five months of treatment. At this stage, switching to letrozole - shown in one study to yield a clinical pregnancy rate of 61.2% compared to 43.0% with clomiphene - or exploring options like gonadotropins or IVF may be more effective than continuing clomiphene indefinitely.
The Future of Personalized Fertility Treatment
Using Genetics to Predict Treatment Response
Advancements in genetics might soon allow clinicians to predict how a woman will respond to fertility treatments like clomiphene. Researchers are studying genetic variants in estrogen receptors (ESR1, ESR2), FSH receptors (FSHR), and drug-metabolizing enzymes such as CYP2D6. The aim is to create polygenic "response scores" that can determine if a woman will ovulate, recommend an ideal starting dose, or suggest alternatives like letrozole.
Current studies show ovulation rates between 59% and 65%, emphasizing that biology plays a significant role beyond the treatment itself. As more genomic data is gathered from historically underrepresented groups, including Black, Hispanic, and Asian women, clinicians may soon classify patients into categories like "likely responders", "dose adjustments needed", or "alternative therapy recommended".
However, genetic testing isn’t yet a standard part of fertility care. Many studies on clomiphene response are small or focus on specific ethnic groups. Additionally, fertility-related genetic tests are rarely covered by insurance, and current treatment guidelines rely on clinical factors like BMI and PCOS phenotype rather than genetic data. To make genetic testing routine, large-scale studies across diverse populations are necessary. These studies would need to link specific genetic variants to live birth outcomes and demonstrate that targeted testing reduces failed cycles and shortens the time to pregnancy.
These genetic advancements could pave the way for more tailored and effective fertility treatments.
Telehealth for Better Access to Fertility Care
Telehealth has become a game-changer in fertility care, breaking down geographic and logistical barriers. Virtual consultations, remote symptom tracking, and mailed lab orders make it easier for women in rural areas or those dealing with transportation, childcare, or rigid work schedules to access care. Companies like Oana Health, which focus on PCOS, weight, and hormone management, are already integrating these tools. They offer services like coordinating local lab testing and shipping medications directly to patients, making fertility care more accessible - especially for uninsured or under-insured women, thanks to transparent pricing models.
By combining telehealth with genetic and metabolic data, these platforms can offer a truly personalized approach. For Black, Hispanic, and low-income women - who often face disparities in fertility care - this model provides earlier evaluations, culturally sensitive education, and ongoing support. It eliminates many of the upfront costs and travel burdens associated with traditional fertility clinics.
This blend of technology and personalization is helping to level the playing field in fertility care.
Closing the Gap in Treatment Outcomes
The future of fertility care lies in merging genetic insights with telehealth to address treatment disparities. A comprehensive model could start with evidence-based protocols tailored to BMI and PCOS, paired with close metabolic management. For women who don’t respond to initial treatments, targeted genetic testing could guide adjustments once it becomes validated and covered by insurance. Telehealth would handle the majority of counseling and follow-ups, reducing both costs and logistical hurdles. Embedding equity goals into treatment plans could further address racial and socioeconomic disparities in outcomes.
By layering genetic data with metabolic care, clinicians can refine treatment strategies. For example, genetic insights could identify women who need higher doses or alternative medications, and treatments like clomiphene could be timed to align with improved metabolic markers. Telehealth platforms that specialize in PCOS and weight management are well-positioned to deliver this integrated approach, coordinating nutrition, exercise, and medication adjustments throughout the treatment process.
This combination of personalized care and improved access is shaping a more equitable future for fertility treatment.
FAQs
How do ethnicity and genetics impact the effectiveness of clomiphene citrate treatment?
Ethnicity and genetics play a crucial role in how people respond to clomiphene citrate. These factors impact hormonal balance and the way the body metabolizes the medication, which can lead to varying levels of treatment success across different ethnic groups.
By recognizing these genetic and ethnic differences, healthcare providers can create more tailored treatment plans aimed at improving individual outcomes. Personalized care strategies, including those provided by specialized telehealth platforms, offer a promising way to address these complexities and enhance patient care.
How does metabolic health impact the effectiveness of clomiphene citrate?
Metabolic health significantly influences how well clomiphene citrate works. Factors such as insulin resistance or obesity can reduce the medication's effectiveness, as they may disrupt the body's response to the treatment.
Taking steps to improve metabolic health - like maintaining a healthy weight, boosting insulin sensitivity, and embracing a balanced lifestyle - can enhance the chances of success. Even small adjustments, such as eating a nutritious diet and staying active, can have a meaningful impact on treatment outcomes.
How can we improve access to fertility care for underserved communities?
Improving access to fertility care for underserved communities means tackling challenges like geographic limitations, high costs, and societal stigma. Telehealth platforms, including services like Oana Health, are helping bridge these gaps by delivering personalized, research-backed treatments that patients can access right from their homes.
Equally important is raising awareness, providing education that respects diverse cultural perspectives, and addressing systemic inequalities within the healthcare system. These efforts are essential to creating fair and inclusive fertility care for everyone.
