Cardiovascular Safety in Long-Term Hormonal Use
Hormonal therapy (HT) remains a key option for managing menopausal symptoms, but its impact on heart health is complex. Here's what you need to know:
- Timing matters: Starting HT before age 60 or within 10 years of menopause may reduce heart risks, but beginning later can increase them.
- Delivery method is critical: Transdermal options (patches, gels) are safer for heart health compared to oral forms, which can raise clotting risks and triglycerides.
- Patient profile influences risk: Factors like obesity, diabetes, and hypertension can increase cardiovascular risks with HT.
- Vaginal estrogen is the safest: For localized symptoms, it avoids systemic risks and is suitable for many high-risk patients.
- HT is not for heart disease prevention: Studies like WHI and HERS show HT doesn't protect against heart disease and may increase certain risks.
FDA updates in 2025 have removed outdated warnings, emphasizing evidence-based, personalized treatment for menopause. Always consult your doctor to assess your risks and options.
Major Study Findings on Hormonal Therapy and Heart Health
The Women's Health Initiative (WHI) Trials
The Women's Health Initiative (WHI) conducted two groundbreaking trials that reshaped how hormonal therapy is viewed in relation to heart health. One trial focused on combined estrogen plus progestin (E+P) using conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA). The other examined estrogen alone (CEE) in women who had undergone hysterectomies.
The E+P trial was halted at 5.2 years due to serious cardiovascular risks, including a 24–29% increase in coronary events during the first year (hazard ratio 1.81) and a significant rise in breast cancer cases. Women taking E+P also faced a 41% higher risk of stroke and a staggering 113% increase in pulmonary embolism. While these risks were statistically modest in absolute terms, they were clinically concerning. On the other hand, the estrogen-alone trial revealed a 35% greater risk of stroke and a 48% higher chance of deep vein thrombosis.
"Estrogen plus progestin does not confer cardiac protection and may increase the risk of CHD among generally healthy postmenopausal women, especially during the first year after the initiation of hormone use." – NEJM
Interestingly, while hormone therapy improved lipid profiles (reducing LDL by 13% and increasing HDL by 7%), it failed to provide overall cardiovascular protection. Follow-up studies continued to refine and expand upon these findings.
HERS and ERA Trials
The HERS trial, which examined hormonal therapy in women with pre-existing coronary disease, built on the WHI findings. Among 2,763 participants, the study found an initial spike in cardiovascular events, followed by a temporary decline that disappeared over the 6.8-year study period.
The trial also highlighted other risks. Women on hormonal therapy had a relative hazard of 2.66 for venous thromboembolism, which remained elevated at 2.08 throughout the study. Additionally, these women were 1.48 times more likely to require gallbladder surgery.
"Postmenopausal hormone therapy should not be used to reduce risk for CHD events in women with CHD." – HERS Research Group
The ERA trial further supported these conclusions, showing that hormone therapy neither slowed coronary atherosclerosis nor prevented recurrent cardiac events. These results mirrored the findings from HERS.
Together, these studies have had a lasting impact on clinical guidelines, making it clear that hormonal therapy should not be prescribed solely for cardiac protection in women with existing cardiovascular conditions.
Hormone Therapy and Cardiovascular Risk
What Affects Cardiovascular Risk in Hormonal Therapy
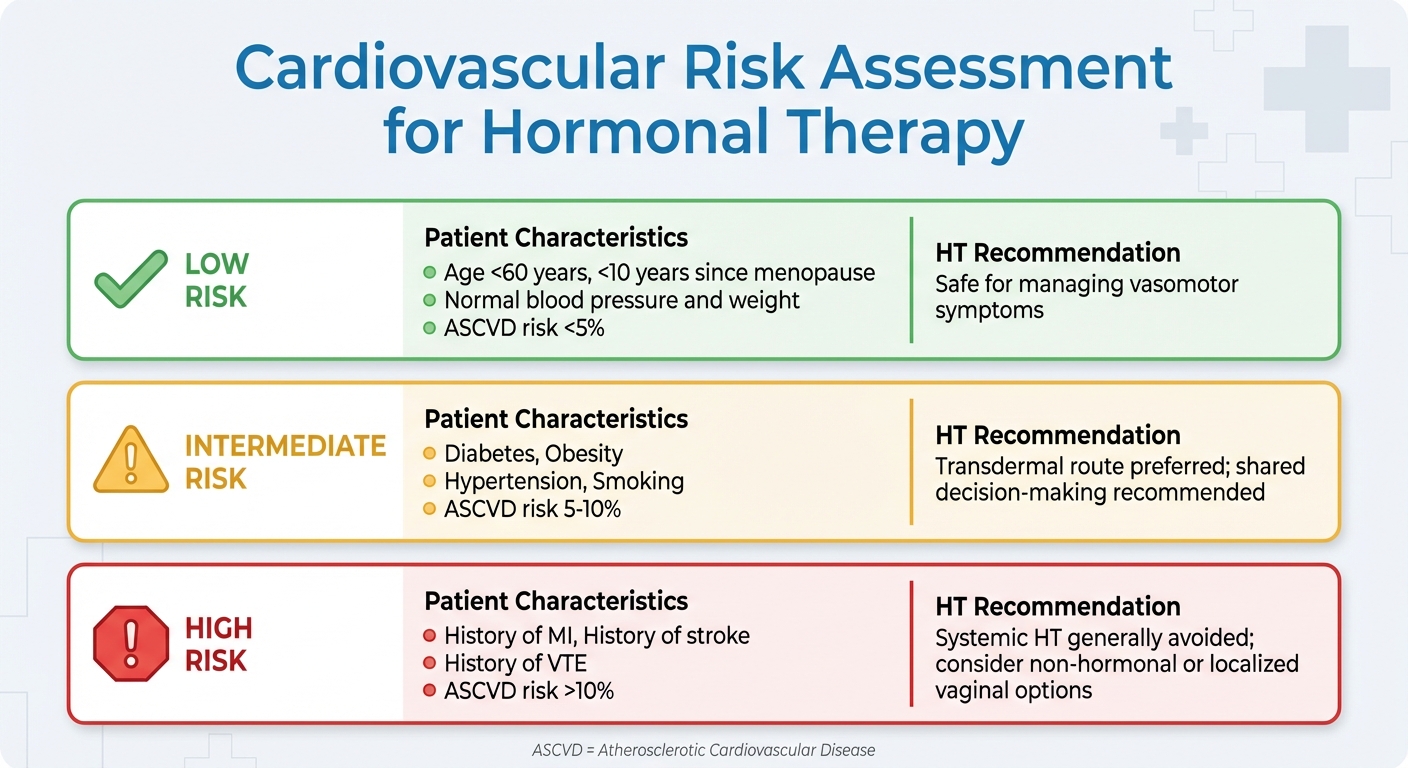
Hormonal Therapy Cardiovascular Risk Assessment by Patient Profile
The Timing Hypothesis
Research has shown that the timing of hormone therapy plays a key role in cardiovascular outcomes. This concept, known as the "Timing Hypothesis", suggests that estrogen's impact on the heart is closely tied to the state of your blood vessels when treatment begins. Starting hormone therapy before age 60 or within 10 years of menopause is often linked to neutral or even positive effects on cardiovascular health. At this stage, estrogen can improve vascular function. However, starting therapy more than 10 to 20 years post-menopause may increase the risk of coronary heart disease and stroke.
A meta-analysis of 19 trials revealed that initiating hormone therapy within 10 years of menopause reduced mortality by 30% (RR 0.70) and cardiac events by 48% (RR 0.52). Similarly, the Women's Health Initiative trials found that women aged 50–59 on estrogen-only therapy showed a trend toward reduced coronary heart disease risk (hazard ratio [HR] 0.60), while those aged 70–79 faced an increased risk (HR 1.09).
"Estrogen may have plaque-destabilizing and other adverse effects in the setting of advanced atherosclerosis, but it provides an appropriate and safe option for treatment of menopausal symptoms when initiated in healthy women <60 years of age or within 10 years of menopause onset." – American College of Cardiology Cardiovascular Disease in Women Committee
The ELITE trial further supported this theory, showing that hormone therapy slowed the progression of subclinical atherosclerosis in women who were less than 6 years from their last menstrual period. However, no benefits were observed in women more than 10 years post-menopause.
Patient Selection and Risk Assessment
In addition to timing, a patient’s overall health profile significantly influences cardiovascular risks associated with hormone therapy. Conditions like hypertension, diabetes, and obesity increase these risks. Women with uncontrolled hypertension (≥180/110 mmHg) face a particularly high stroke risk. While hormone therapy may improve insulin sensitivity in diabetic women, it does not lower their elevated cardiovascular mortality risk, which is two to three times higher than average.
Obesity also amplifies risks, especially with oral hormone therapy. Data from the Women's Health Initiative trials showed that oral systemic hormone therapy increased the risk of venous thromboembolism threefold in overweight women and nearly sixfold in obese women compared to their lean counterparts.
To manage these risks, clinicians now adopt a risk-stratified approach when considering hormone therapy. Low-risk patients - those under 60, within 10 years of menopause, with normal blood pressure and weight, and a 10-year ASCVD (atherosclerotic cardiovascular disease) risk below 5% - are generally suitable candidates. For intermediate-risk patients, such as those with diabetes, obesity, or a 10-year ASCVD risk between 5% and 10%, transdermal formulations are often preferred. High-risk patients, including those with a history of heart attack, stroke, venous thromboembolism, or a 10-year ASCVD risk above 10%, are typically advised to avoid systemic hormone therapy.
| Risk Category | Patient Characteristics | HT Recommendation |
|---|---|---|
| Low Risk | <60 years old, <10 years since menopause, normal BP/weight, ASCVD risk <5% | Safe for managing vasomotor symptoms |
| Intermediate Risk | Diabetes, obesity, hypertension, smoking, ASCVD risk 5–10% | Transdermal route preferred; shared decision-making recommended |
| High Risk | History of MI, stroke, VTE, or ASCVD risk >10% | Systemic HT generally avoided; consider non-hormonal or localized vaginal options |
These individualized assessments guide clinicians in making informed decisions to ensure hormone therapy is used safely and effectively.
sbb-itb-6dba428
How Delivery Method Affects Heart Safety
The way hormones are delivered can significantly influence cardiovascular health, making it an important consideration for optimizing safety.
Oral vs. Transdermal Hormonal Therapy
The choice between oral and transdermal hormonal therapy has a notable impact on cardiovascular risk. Oral estrogen undergoes processing in the liver (first-pass hepatic metabolism), which increases the production of clotting factors and other substances that promote blood clots, raising the risk of conditions like venous thromboembolism (VTE) and stroke. On the other hand, transdermal formulations (such as patches or gels) bypass the liver entirely, reducing their impact on coagulation and inflammatory markers.
Research backs up these differences. The ESTHER study found that women on oral estrogen had an odds ratio of 4.2 for developing VTE, compared to an odds ratio of just 0.9 for those using transdermal estrogen - essentially no increased risk relative to non-users. Similarly, data from the Women’s Health Initiative showed that oral estrogen-only therapy led to 79 additional stroke cases and 77 more blood clot cases per 10,000 women over 7.2 years. A 2024 Swedish study further highlighted that oral continuous estrogen-progestin therapy carried a hazard ratio of 1.61 for VTE, while sequential oral therapy had an even higher ratio of 2.00.
"Transdermally administered estrogen has little or no effect in elevating prothrombotic substances and may have beneficial effects on proinflammatory markers." – American College of Obstetricians and Gynecologists
Women with existing risk factors, such as a BMI over 30 kg/m², a history of blood clots, or genetic clotting disorders, are strongly advised to choose transdermal methods due to their safer profile. Additionally, oral estrogens are known to increase triglyceride levels by 5% to 15% and elevate C-reactive protein, whereas transdermal options have minimal effects on these markers.
For women seeking an even safer alternative, localized therapies offer distinct advantages.
Vaginal Estrogen Safety
When it comes to cardiovascular safety, low-dose vaginal estrogen is the top choice among delivery methods. Its systemic absorption is minimal, keeping circulating estrogen levels within the normal postmenopausal range. As a result, it doesn’t trigger the coagulation or inflammatory changes often associated with oral estrogen.
Observational studies confirm that vaginal estrogen therapy does not increase the risk of cardiovascular disease, stroke, or blood clots. This makes it an excellent option for women who cannot use systemic hormone therapy, including those with a history of cardiovascular disease, stroke, or VTE.
"Given minimal systemic absorption, low-dose vaginal ET is an option for women for whom systemic HT may be contraindicated, including those with a history of estrogen-responsive cancers, CVD, stroke, or VTE." – American College of Cardiology Cardiovascular Disease in Women Committee
It’s worth noting that the FDA’s "black box" warning on vaginal estrogen products is based on studies involving high-dose systemic hormones, not low-dose vaginal formulations. Experts emphasize that this warning does not reflect the safety profile of low-dose vaginal estrogen. Common low-dose options include estradiol tablets (10 μg), vaginal rings (7.5 μg/24h), and various creams.
Clinical Guidelines and Treatment Recommendations
Clinical guidelines have evolved to address the risks associated with hormonal therapy, offering clear recommendations for its safe use.
The American Heart Association (AHA) and American College of Cardiology (ACC) emphasize the importance of risk stratification using the Pooled Cohort Equations (PCE) before initiating hormonal therapy. Additional risk factors, such as a family history of premature atherosclerotic cardiovascular disease (ASCVD), chronic inflammatory conditions, and preeclampsia, should also be considered.
"It is appropriate that no medical societies currently recommend HT for the primary or secondary prevention of cardiovascular disease"
Risk Assessment and When to Avoid Hormonal Therapy
There are specific scenarios where systemic hormonal therapy should be entirely avoided. Absolute contraindications include a history of venous thromboembolism (VTE), pulmonary embolism, stroke, transient ischemic attack (TIA), myocardial infarction, or estrogen-sensitive cancers.
"Because we believe coronary artery dissection is hormone-driven, patients with this condition should never take oral estrogen therapy"
Uncontrolled hypertension - defined as blood pressure at or above 180/110 mm Hg - is considered a relative contraindication. Hormonal therapy in such cases should only be considered after blood pressure is well-controlled.
These risk assessments guide clinicians in identifying safer treatment pathways.
Choosing Lower-Risk Treatment Options
For women who are eligible for hormonal therapy, selecting the appropriate delivery method is crucial. Transdermal formulations, such as patches and gels, are often the preferred choice for women with intermediate cardiovascular risk or those with obesity, a smoking history, or metabolic concerns.
"The clear winner in this updated review of treatment for vasomotor symptoms in menopause is transdermal formulations of HT"
Unlike oral options, transdermal delivery bypasses the liver, which helps maintain neutral triglyceride levels and significantly reduces the risk of VTE.
Women with hypertriglyceridemia should avoid oral hormonal therapy, as it can elevate triglyceride levels by 5% to 15%. In contrast, transdermal formulations can reduce triglycerides by 5% to 30%. For women with an intact uterus, it's essential to include a progestogen - preferably micronized progesterone - alongside estrogen therapy to prevent endometrial hyperplasia.
Regular monitoring of cardiovascular health is critical. For instance, oral conjugated equine estrogen has been shown to raise systolic blood pressure by 1–1.5 mm Hg. Healthcare providers should routinely reassess the therapy's risks and benefits, aiming to use the lowest effective dose for the shortest time necessary.
Conclusion
Hormonal therapy, when tailored to an individual’s health profile, can be a safe option for long-term cardiovascular health. Timing plays a major role - starting therapy early, particularly before age 60 or within 10 years of menopause, has been shown to cut cardiovascular disease risk by up to 50%. This "window of opportunity" is essential not only for managing menopause symptoms but also for supporting heart health in the long run.
The delivery method also matters. Transdermal options, like patches, gels, and sprays, are often better suited for women with metabolic issues, obesity, or high triglycerides. Compared to oral formulations, transdermal therapies are associated with lower triglyceride levels and reduced risks of venous thromboembolism and stroke.
In a significant development, the FDA removed "black box" warnings from hormone replacement therapy products in November 2025. This decision marked a shift toward evidence-based care. As FDA Commissioner Marty Makary, M.D., M.P.H., noted:
"Tragically, tens of millions of women have been denied the life-changing and long-term health benefits of hormone replacement therapy because of a medical dogma rooted in a distortion of risk".
This regulatory update reflects decades of research showing no notable difference in all-cause or cardiovascular mortality between women on hormone therapy and those on a placebo. It underscores the importance of personalized treatment plans that consider individual risk factors.
Personalized risk assessment remains critical for guiding therapy decisions. Tools like the 10-year ASCVD risk score (where <5% indicates low risk) and Coronary Artery Calcium scoring help healthcare providers identify the safest and most effective options for each woman. For those seeking expert care, Oana Health offers telehealth services, connecting women with licensed professionals who specialize in science-based, tailored hormone therapy delivered conveniently to their homes.
FAQs
What is the safest way to take hormonal therapy for cardiovascular health?
For lowering cardiovascular risks, non-oral delivery methods such as transdermal patches, gels, or vaginal preparations (specifically for estrogen) are often the safest choices. These options avoid passing through the liver, which helps reduce potential effects on blood clotting and cholesterol levels.
If you're thinking about hormonal therapy, make sure to talk with a licensed healthcare provider. They'll help you choose the option that best fits your health and individual needs.
Does the timing of starting hormone therapy impact heart health?
Yes, when you start hormone therapy can play a big role in your cardiovascular health. Studies indicate that beginning hormone therapy during perimenopause or within about 10 years after menopause (usually before age 60) is associated with a reduced risk of heart disease. On the other hand, starting hormone therapy many years after menopause might raise the chances of developing cardiovascular problems.
If you're thinking about hormone therapy, make sure to talk to a licensed healthcare provider. They can help you figure out the safest and most suitable option based on your unique health needs.
Why isn’t hormonal therapy recommended to prevent heart disease?
Hormonal therapy is not recommended for preventing heart disease. Research has shown that it does not reduce the risk of cardiovascular events. In fact, some studies indicate it may raise the likelihood of heart disease, stroke, and blood clots.
Although hormonal treatments can be helpful for addressing other health issues, relying on them to prevent heart-related conditions is neither safe nor effective. If you’re considering hormonal therapy or have questions about your heart health, it’s best to consult a licensed medical professional for advice tailored to your needs.
