Emerging Biomarkers for Adrenal Hyperandrogenism
Adrenal hyperandrogenism, a condition where the adrenal glands produce excessive androgens, is linked to disorders like Congenital Adrenal Hyperplasia (CAH), premature adrenarche, and Polycystic Ovary Syndrome (PCOS). Symptoms include hirsutism, acne, and menstrual irregularities, but diagnosing this condition has been challenging due to inconsistent results from standard tests like DHEAS and 17-hydroxyprogesterone (17OHP).
Recent research highlights 11-oxygenated androgens (e.g., 11-ketotestosterone) and 21-deoxycortisol as more precise biomarkers. These markers, measurable through advanced techniques like LC-MS/MS, provide better insights into adrenal activity and help distinguish adrenal-driven from ovarian-driven androgen excess. This can improve diagnosis and treatment, particularly for conditions like non-classic CAH and PCOS.
Key developments include:
- 11-ketotestosterone: Dominant androgen in 21-hydroxylase deficiency, reflecting adrenal activity.
- 21-deoxycortisol: Highly specific for non-classic CAH with 100% sensitivity.
- LC-MS/MS testing: Offers accurate steroid profiling, overcoming limitations of older methods.
Emerging tools like hair follicle analysis and miRNA profiling also show promise for non-invasive and precise diagnostics. These advancements are enabling more targeted treatments, such as glucocorticoid suppression for adrenal-driven PCOS and tailored therapies based on individual androgen profiles.
New Biomarkers for Adrenal Hyperandrogenism
Traditional vs New Biomarkers for Adrenal Hyperandrogenism Diagnosis
11-Oxy Androgen Pathway Biomarkers
Scientists have pinpointed adrenal-specific 11-oxygenated C19 (11oxC19) steroids as key markers. These include compounds like 11β-hydroxyandrostenedione, 11-ketoandrostenedione, 11β-hydroxytestosterone, and 11-ketotestosterone. Unlike testosterone and androstenedione, these biomarkers are exclusively linked to adrenal activity.
Among these, 11-ketotestosterone (11KT) and 11-ketodihydrotestosterone (11KDHT) stand out due to their biological activity, which mirrors that of testosterone and dihydrotestosterone (DHT). In fact, 11KT’s potency is only about five times lower than testosterone . For individuals with 21-hydroxylase deficiency, 11KT levels are roughly double those of testosterone, making it the primary circulating androgen in these cases. Even in healthy young men, 11-oxy androgens contribute about 22% of the total free androgen pool.
"11-ketotestosterone and its 5α-reduced metabolite, 11-ketodihydrotestosterone are potent agonists of the human androgen receptor, similar to the classic androgens testosterone and dihydrotestosterone." - Adina F. Turcu, M.D., Division of Metabolism, Endocrinology and Diabetes
Interestingly, while traditional androgens tend to decline with age, 11-oxy androgens either remain steady or even increase after menopause . In individuals with classic 21-hydroxylase deficiency, levels of 11oxC19 steroids are three to four times higher than in healthy individuals .
These direct adrenal markers are complemented by other androgen-synthesis pathways that can also contribute to hyperandrogenism.
The Backdoor Pathway in Androgen Production
In addition to direct adrenal markers, an alternative route, known as the "backdoor pathway", sheds light on androgen excess. This pathway bypasses common intermediates like DHEA and androstenedione, instead producing potent androgens through a different process. In cases of 21-hydroxylase deficiency, 17-hydroxyprogesterone builds up and is redirected into this pathway, leading to the production of DHT via intermediates such as androsterone. This pathway is particularly active during fetal development and early infancy, serving as a major source of DHT in newborns with 21-hydroxylase deficiency.
A standout marker in this context is 21-deoxycortisol (21dF), which is highly specific for 21-hydroxylase deficiency. It forms when the enzyme CYP11B1 acts on accumulated 17-hydroxyprogesterone . Notably, basal follicular levels of 21-deoxycortisol at or above 0.087 ng/mL can identify non-classic adrenal hyperplasia with 100% sensitivity. This marker also shows a strong correlation with ACTH levels (r ≈ 0.7, p < 0.0001) and adrenal volume as measured by CT scans .
"21-deoxycortisol, which are all P450 11B1 products, showed the tightest correlations with adrenal volume as assessed by computed tomography." - Adina F. Turcu, Division of Metabolism, Endocrinology and Diabetes, University of Michigan
Unlike 17-hydroxyprogesterone, which can fluctuate significantly with glucocorticoid treatment, 21-deoxycortisol provides a more stable measure, making it especially useful for monitoring long-term treatment .
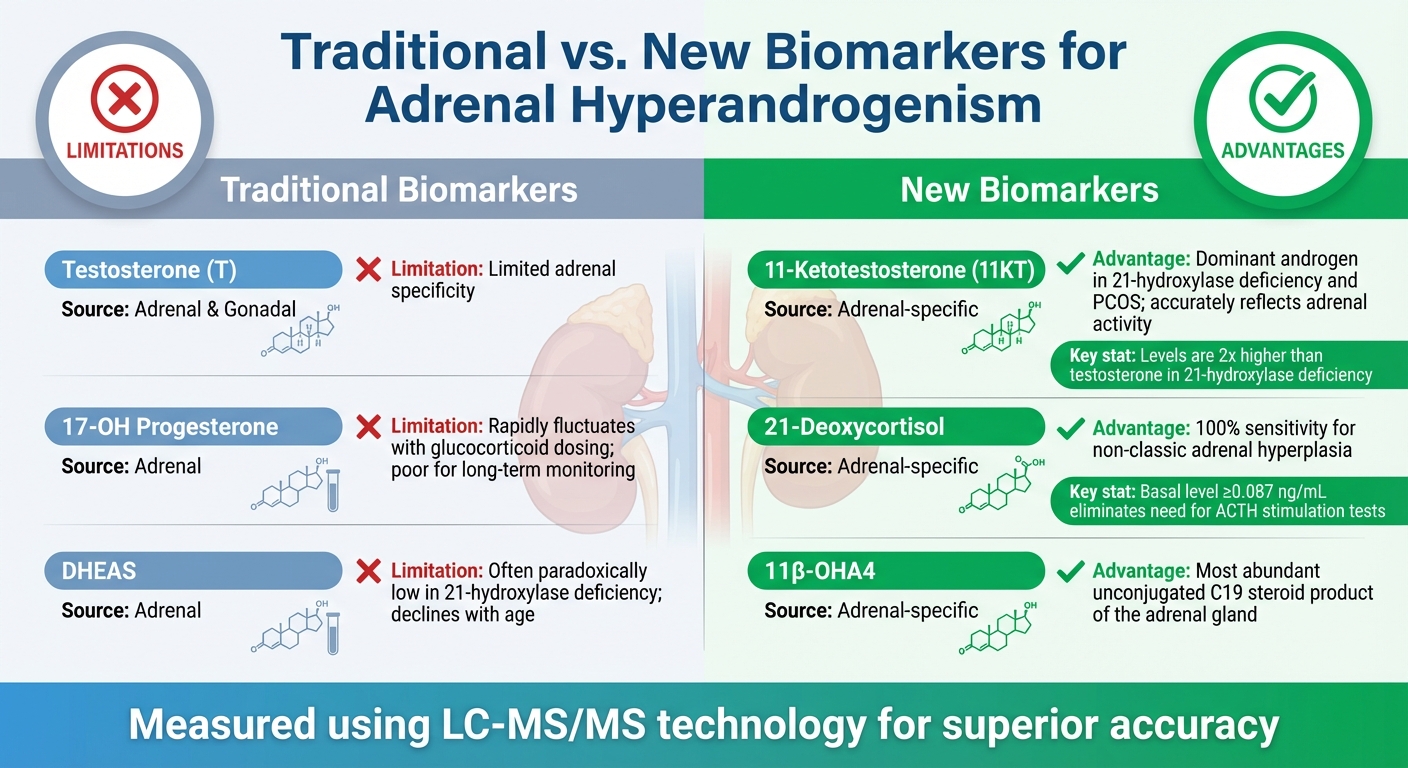
Traditional vs. New Biomarkers
The table below contrasts traditional adrenal biomarkers with emerging ones, highlighting the diagnostic improvements offered by the newer markers:
| Biomarker | Type | Source | Clinical Limitations/Advantages |
|---|---|---|---|
| Testosterone (T) | Traditional | Adrenal & Gonadal | Limited adrenal specificity |
| 17-OH Progesterone | Traditional | Adrenal | Rapidly fluctuates with glucocorticoid dosing; poor for long-term monitoring |
| DHEAS | Traditional | Adrenal | Often paradoxically low in 21-hydroxylase deficiency; declines with age |
| 11-Ketotestosterone | New | Adrenal-specific | Dominant androgen in 21-hydroxylase deficiency and PCOS; accurately reflects adrenal activity |
| 21-Deoxycortisol | New | Adrenal-specific | 100% sensitivity for non-classic adrenal hyperplasia; eliminates need for ACTH stimulation tests |
| 11β-OHA4 | New | Adrenal-specific | Most abundant unconjugated C19 steroid product of the adrenal gland |
A key factor in these advancements has been the adoption of Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). This method addresses the cross-reactivity issues found in traditional immunoassays and provides the precision needed to measure 11-oxyandrogens accurately .
Multi-Omics Research Findings
Metabolomics and Transcriptomics Studies
Cutting-edge technologies like LC-MS/MS and UPC2-MS/MS now allow scientists to measure dozens of steroids at once with far greater sensitivity than older methods.
In untargeted metabolomics research using UPLC-IMS-HR-MS/MS, researchers identified 7,968 unique features and 276 metabolites in mouse adrenal tissue. Meanwhile, transcriptomic profiling unveiled zone-specific gene expression patterns that explain differences in steroid production.
Single-sample multi-omics techniques have taken this a step further by analyzing steroids, catecholamines, and TCA cycle intermediates in tandem, while also preserving native proteins. This approach uncovered a 23-fold increase in progesterone levels in mouse adrenal tissues when using a specialized cell disruption buffer compared to conventional methods.
"This novel multi-omics approach not only minimizes the amount of sample required and overcomes problems associated with tissue heterogeneity, but also provides a more complete picture of adrenal function." - Nicole Bechmann, Institute of Clinical Chemistry and Laboratory Medicine, Technische Universität Dresden
There’s also growing interest in alternative testing materials. For example, hair follicle analysis offers a retrospective view of steroid exposure, with each centimeter of hair representing about one month of systemic steroid levels. This method sidesteps the diurnal fluctuations that often complicate blood testing.
In addition to these biochemical approaches, genetic markers are emerging as another promising avenue for diagnostics.
miRNA Research Findings
Beyond metabolism and transcriptomics, genetic regulators like microRNAs (miRNAs) are adding new layers of insight into adrenal function. These small molecules, which control gene expression, play a key role in adrenal hormone production. Specific miRNAs - such as miR-96, miR-101a, miR-142-3p, and miR-433 - target the glucocorticoid receptor gene (Nr3c1), influencing how the adrenal gland responds to ACTH stimulation. Studies have shown these miRNAs can reduce glucocorticoid receptor activity by 20–40%.
"ACTH stimulation could be demonstrated to acutely influence adrenal miR expression pattern in vivo; thus, potentially modulating adrenal response to acute stressors." - Anna Riester, Ludwig-Maximilians-Universität München
From a clinical perspective, miR-483-5p has shown exceptional diagnostic promise. Its serum levels are 24 times higher in patients with active adrenocortical carcinoma compared to those with benign tumors, with an 81.3% sensitivity and 88% specificity. Similarly, miR-22-3p has proven effective in distinguishing Cushing syndrome from non-functioning adrenal tumors, achieving an Area Under the Curve of 0.800.
One of the most appealing aspects of circulating miRNAs is their stability in body fluids, which eliminates the need for invasive tissue sampling. For example, in Primary Bilateral Macronodular Adrenal Hyperplasia, researchers identified 135 differentially expressed miRNAs - including hsa-miR-1180-3p and hsa-miR-4732-5p - that can help differentiate hyperplasia from other types of adrenocortical adenomas.
sbb-itb-6dba428
Clinical Testing and Medical Applications
Laboratory Testing of New Biomarkers
With advancements in multi-omics research, clinical labs are now fine-tuning biomarker testing to provide more accurate treatment guidance. Traditional immunoassays are being replaced by cutting-edge techniques like Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) and Ultra-Performance Convergence Chromatography-Tandem Mass Spectrometry (UPC2-MS/MS). These methods offer greater precision, allowing for the simultaneous measurement of multiple steroids, which is especially important given the low levels of many circulating androgens.
Research using specialized cell models highlights the potential of the 11KT to testosterone (T) ratio as a reliable marker for monitoring disease control in 21-hydroxylase deficiency (21OHD).
"The ratio of 11KT/T could serve as an excellent clinical indicator of disease control in patients with 21OHD, particularly in post-pubertal males." - Adina F. Turcu and Richard J. Auchus
For non-classic adrenal hyperplasia (21-NCAH), basal follicular testing using LC-MS/MS has shown impressive diagnostic accuracy. In fact, a 21-deoxycortisol level of ≥ 0.087 ng/mL was found to have 100% sensitivity in identifying 21-NCAH in women presenting PCOS-like symptoms.
Clinical Trials and Treatment Options
The discovery of adrenal-specific biomarkers is opening doors to more targeted treatments. For instance, women with elevated levels of 11β-hydroxyandrostenedione (11OHA4) and 11-ketoandrostenedione (11KA4) tend to have distinct metabolic profiles, such as higher BMI and HOMA-IR scores. This allows clinicians to better assess metabolic risks and tailor insulin-sensitizing therapies accordingly.
"Adrenal C11-oxy steroids have the potential of being used diagnostically to identify younger women and adolescents with PCOS who also have some evidence of micronodular adrenocortical hyperplasia." - Amanda C. Swart et al.
Clinical trials are also investigating the use of P450c17 inhibitors, like abiraterone acetate, to suppress adrenal androgen precursors. These treatments are being explored for conditions such as castration-resistant prostate cancer and severe hyperandrogenism. For patients with 21-NCAH identified through these advanced biomarkers, glucocorticoid suppression is emerging as a targeted alternative to conventional PCOS therapies. Moreover, the androstenedione to 11β-hydroxyandrostenedione (A:11β-OHA) ratio has proven highly effective, with 100% sensitivity and 84% specificity in identifying adrenal hyperandrogenism. This provides a reliable tool for distinguishing adrenal from ovarian sources of excessive androgens.
These advancements are paving the way for more tailored approaches to managing PCOS.
Personalized PCOS Treatment
The precision offered by 11-oxygenated androgen measurements is transforming how PCOS is managed. These biomarkers help differentiate between adrenal-driven and ovarian-driven hyperandrogenism, which is critical since treatment strategies vary significantly. For example, women with elevated adrenal androgens often benefit from glucocorticoid suppression, while those with ovarian hyperandrogenism typically respond better to oral contraceptives or anti-androgens like spironolactone. By pinpointing the source of androgen excess, doctors can reduce the trial-and-error process and provide faster symptom relief.
Interestingly, research has shown that 11-oxygenated C19 steroids are the dominant circulating androgens in many women with PCOS, challenging the traditional reliance on testosterone and DHEAS tests. This explains why some women experience severe androgenic symptoms despite having "normal" testosterone levels on standard tests.
For personalized and science-backed treatments addressing PCOS-related concerns like acne, hair loss, and unwanted facial hair, Oana Health (https://oanahealth.com) offers solutions delivered by licensed medical professionals. These advancements are helping patients achieve better outcomes with treatments tailored to their unique profiles.
The Future of Adrenal Hyperandrogenism Biomarkers
The move toward steroidomics - analyzing a broad range of steroids using LC–MS/MS - is revolutionizing how adrenal hyperandrogenism is diagnosed and treated. Unlike traditional immunoassays, which often suffer from cross-reactivity and overestimation, LC–MS/MS can detect low-abundance adrenal steroids that older methods frequently miss. For example, basal 21-deoxycortisol levels of at least 0.087 ng/mL have shown 100% sensitivity for diagnosing 21-NCAH, potentially reducing the reliance on ACTH stimulation tests. These advancements in laboratory techniques are being complemented by non-invasive diagnostic methods, making testing more accessible.
Non-invasive approaches, such as hair follicle analysis, are providing new ways to monitor hormone levels over time. This method offers a retrospective view of hormone fluctuations spanning several months, avoiding the influence of diurnal variations and acute stress that can skew single-point blood tests. Such innovations are particularly useful for remote monitoring and long-term management.
These technological breakthroughs are also finding synergy with telehealth platforms, which are expanding access to specialized care. Patients no longer need to travel to endocrinology clinics to benefit from advanced hormone testing and personalized treatment plans. For instance, women dealing with PCOS-related symptoms, such as acne, hair thinning, or excessive facial hair, can now leverage services like Oana Health. This platform provides science-backed treatments prescribed by licensed professionals and delivers them directly to patients' homes, aligning with the trend toward more personalized PCOS care.
Despite these advancements, challenges persist. The high cost and technical demands of LC–MS/MS limit its routine use in clinical settings, and promising biomarkers like 11-ketotestosterone are not yet widely available as standard tests, even though they hold significant diagnostic potential. As these technologies become more accessible and affordable, the combination of precise biomarker testing with telehealth services will open the door to more personalized and effective treatment options, setting the stage for a new era in care.
FAQs
What makes 11-oxygenated androgens more effective than traditional biomarkers in diagnosing adrenal hyperandrogenism?
11-oxygenated androgens are adrenal steroids that offer a dependable way to measure adrenal androgen excess. Unlike traditional markers such as DHEA-S, androstenedione, or 17-OHP, these androgens maintain consistent levels regardless of a person’s age, making them an age-independent diagnostic option.
This consistency is particularly useful when traditional biomarkers yield uncertain or conflicting results. By providing a more accurate view of adrenal function, 11-oxygenated androgens can play a key role in diagnosing and tailoring treatment plans for conditions like adrenal hyperandrogenism.
How does LC-MS/MS enhance the accuracy of diagnosing conditions like non-classic CAH and PCOS?
LC-MS/MS, or liquid chromatography-tandem mass spectrometry, enhances diagnostic precision by measuring multiple hormones at once with exceptional accuracy. It can analyze hormones like testosterone, androstenedione, DHEA-S, and adrenal-derived 11-oxygenated androgens, all while minimizing interference from other substances. This results in a clearer and more reliable hormone profile.
In cases of non-classic congenital adrenal hyperplasia (CAH), LC-MS/MS plays a key role in detecting markers such as elevated 17-OHP, progesterone, and 21-deoxycortisol. It’s equally effective in evaluating hyperandrogenism in PCOS patients, providing detailed insights into hormonal imbalances. This level of detail allows for more precise diagnoses and tailored treatment plans.
How do microRNAs (miRNAs) affect adrenal hormone production?
MicroRNAs (miRNAs) are tiny RNA molecules that don’t code for proteins but play a crucial role in regulating adrenal hormone production. They do this by controlling the expression of specific genes within the adrenal glands. For instance, miRNAs influence enzymes and proteins like CYP11B2 (associated with aldosterone production), CYP17A1 (important for androgen precursors), and StAR (essential for steroid production). This regulation directly affects hormones such as aldosterone, cortisol, and adrenal androgens, all of which are vital for managing blood pressure, maintaining electrolyte balance, and addressing symptoms linked to hyperandrogenism.
Recent studies have uncovered distinct miRNA patterns tied to changes in hormone levels. Notably, miRNAs like miR-483-5p and miR-210 have emerged as potential biomarkers for conditions such as primary aldosteronism and adrenal hyperplasia. These discoveries could pave the way for more precise diagnostic methods and treatments tailored to hormone-related health issues.
Oana Health incorporates this advanced research into its personalized telehealth services. By offering prescription-based treatments, they address symptoms caused by excess adrenal androgens, including unwanted facial hair, acne, and other related concerns.
