Top FDA Labeling Changes for Hormonal Medications
The FDA made major updates to hormonal medication labels in November 2025, removing outdated black box warnings for cardiovascular disease, breast cancer, and dementia. These updates aim to improve access to treatments like menopausal hormone therapy (MHT) and testosterone products while ensuring safety and personalized care. Here's what changed:
- Black box warnings removed: Cardiovascular, breast cancer, and dementia risks are no longer emphasized for MHT. Evidence shows benefits when therapy starts before age 60 or within 10 years of menopause.
- Dosing guidance updated: No longer limited to the lowest dose for the shortest time, allowing longer use based on individual needs.
- Local estrogen therapy clarified: Labels now exclude systemic risk warnings, encouraging use for genitourinary symptoms.
- Testosterone products revised: Cardiovascular risk warnings replaced with information about increased blood pressure.
These changes reflect updated research and aim to reduce fear around hormone therapy, making treatment more accessible for millions of women. Patients should consult healthcare providers to discuss these updates and explore options tailored to their needs.
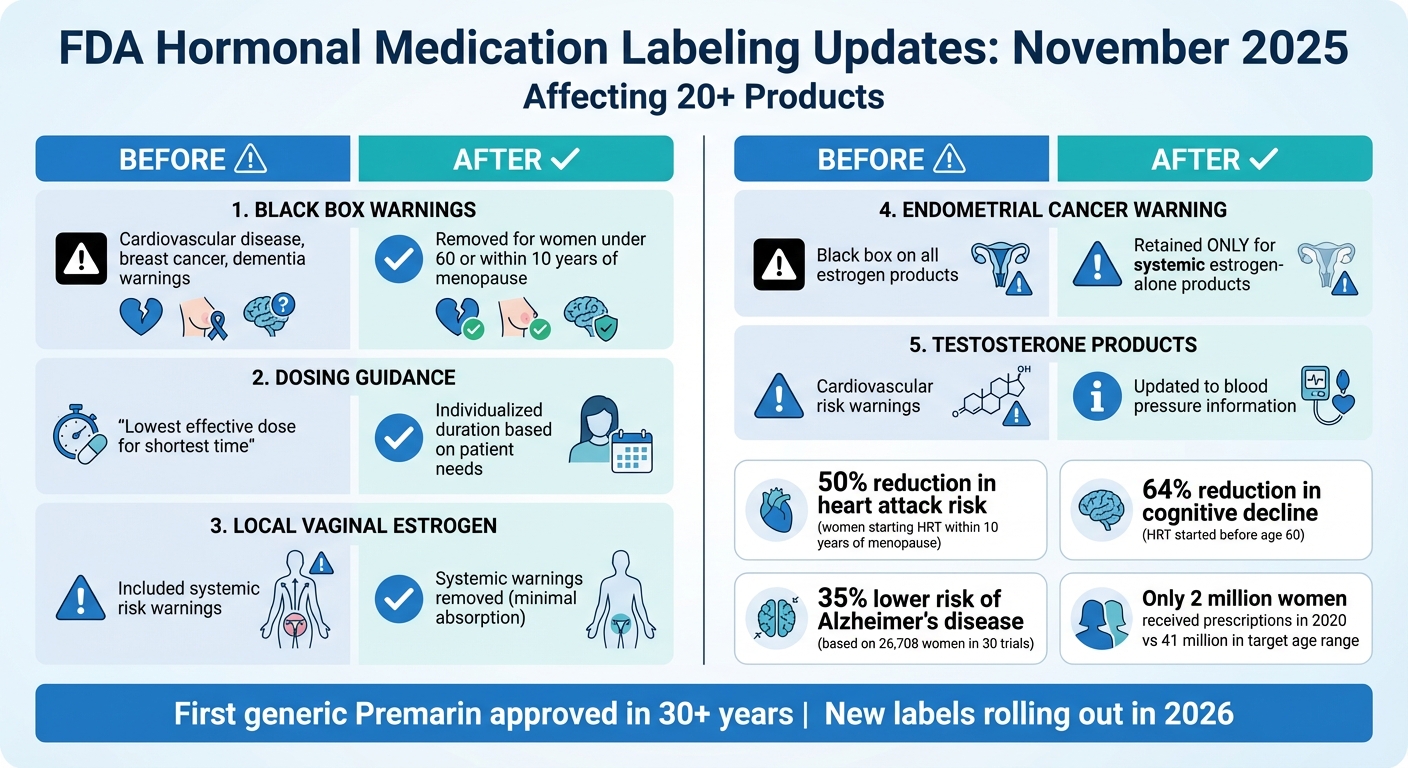
FDA Hormonal Medication Labeling Changes November 2025: Before vs After
FDA head explains decision to drop 'black box' warnings from menopause hormone treatment

Major FDA Labeling Updates for Hormonal Medications
The FDA has introduced updated labeling guidelines for hormonal medications, aiming to provide more precise, age-specific recommendations rather than relying on outdated, generalized warnings. These changes, effective November 2025, impact over 20 products containing estrogen and progestogen. This shift is designed to improve how hormonal therapies are communicated to both patients and healthcare providers. Below, we break down the updates related to cardiovascular, cognitive, and endometrial risks.
Removal of Black Box Warnings for Cardiovascular Disease and Breast Cancer
One major change is the removal of black box warnings for cardiovascular disease and breast cancer from menopausal hormone therapy products. This decision comes after a reassessment of the Women's Health Initiative (WHI) study data, which was based on an older population (average age 63) and outdated hormone formulations, making it less relevant to current practices.
The WHI study's findings on breast cancer risk were not statistically significant, showing fewer than one additional case of breast cancer per year per 1,000 women. Interestingly, women taking estrogen-only pills were found to be less likely to develop or die from breast cancer. For cardiovascular health, women who started hormone replacement therapy (HRT) within 10 years of menopause or before age 60 experienced a 50% reduction in heart attack risk.
"The black box is really one size fits all. It scares everyone away. Without the black box warning there may be more focus on the actual findings, how they differ by age and underlying health factors".
Dr. JoAnn Manson from Harvard Medical School highlighted how the original warnings created unnecessary fear, overshadowing age-specific benefits. The updated labeling encourages a more individualized approach, focusing on the timing of therapy initiation to assess cardiovascular advantages.
Removal of Dementia-Related Warnings
The FDA has also removed dementia-related warnings from prescribing information. This update reflects a better understanding of how hormone therapy impacts cognitive health depending on the age at which it is started.
A recent review of 30 trials involving 26,708 women revealed that HRT use can lead to a 64% reduction in cognitive decline and a 35% lower risk of Alzheimer's disease when initiated before age 60. These benefits are only observed when therapy begins within the "window of opportunity", defined as the first 10 years after menopause.
Continued Endometrial Cancer Warnings for Specific Products
Despite these updates, the FDA has retained the black box warning for endometrial cancer on systemic estrogen-alone products. This warning remains critical because estrogen without progestogen increases the risk of uterine cancer in women with an intact uterus. However, this warning has been removed from local vaginal estrogen products and combination estrogen-progestogen therapies, as the inclusion of progestogen offers protective benefits.
For women using systemic estrogen-alone therapy, regular monitoring with their healthcare provider remains essential to manage endometrial cancer risks. This targeted approach reflects the FDA's effort to balance safety with the removal of outdated warnings, ensuring that critical information is preserved where it is most relevant while encouraging appropriate treatment options.
Simplified Labeling for Specific Hormonal Therapies
In November 2025, the FDA introduced updates aimed at making safety information for hormonal therapies more relevant and less alarming. These changes reflect a nuanced understanding that not all hormonal therapies carry the same risks. By tailoring labels to the specific uses and effects of different formulations, the FDA is paving the way for more precise guidance.
Updated Guidance for Younger Menopausal Women
One significant update focuses on recommendations for women under 60 or those within 10 years of menopause onset. This adjustment addresses a long-standing gap, as the original Women's Health Initiative (WHI) primarily included women averaging 63 years old, while the typical age for menopause in the U.S. is closer to 51. The new labels incorporate WHI data for women aged 50–59, aligning the guidance more closely with the average age of menopause. This shift supports more personalized and extended treatment options.
Another key change is the removal of the "lowest effective dose for the shortest time" guideline. Women can now stay on therapy for longer periods if their individual benefits outweigh the risks, eliminating the pressure of arbitrary time limits. This update may help remove barriers that previously discouraged women from seeking or continuing therapy.
Changes to Local Vaginal Estrogen Product Labels
While systemic therapies now include age-specific recommendations, local treatments have also seen important updates. The FDA has simplified labels for local vaginal estrogen products - such as creams, rings, and tablets - by focusing only on risks relevant to these formulations. As these products involve minimal systemic absorption, their labels no longer include boxed warnings for cardiovascular disease, breast cancer, dementia, or endometrial cancer.
"Removing the scary boxed warning from local vaginal estrogen is a victory for women's health and for those who care for and about menopausal women." - Dr. JoAnn Pinkerton, Professor of Obstetrics and Gynecology, University of Virginia
This change aims to reassure women about the safety of treating genitourinary syndrome, encouraging them to seek relief without unnecessary fear.
sbb-itb-6dba428
What These Changes Mean for Patients and Access to Care
The FDA's updated labeling for hormonal medications marks a major shift in how these treatments are prescribed and accessed in the U.S. These updates aim to lower long-standing barriers that have kept millions of women from receiving effective care. By addressing both clinical practices and patient experiences, these changes could have a meaningful impact on access to treatment.
Effects on Prescribing Practices
Healthcare providers now have more flexibility to prescribe hormone therapy based on individual patient needs rather than rigid, one-size-fits-all guidelines. The removal of the "lowest effective dose for the shortest amount of time" recommendation allows clinicians to extend therapy duration when the benefits outweigh the risks. This change is critical, especially considering that in 2020, only about 2 million women received systemic hormone therapy prescriptions, despite 41 million women being in the age range most affected by menopausal symptoms.
"The updated labels will better allow patients and clinicians to engage in a shared decision-making process, without an unnecessary barrier, when it comes to treatment of menopausal symptoms." - Steven J. Fleischman, President, American College of Obstetricians & Gynecologists
Additionally, the elimination of boxed warnings for local vaginal estrogen clarifies that systemic risks do not apply, removing a key hurdle for prescribing these treatments. These changes not only influence clinical decisions but also encourage greater patient involvement in their care.
Better Patient Understanding and Confidence
For years, boxed warnings discouraged many women from considering hormone therapy. By removing warnings related to cardiovascular disease, breast cancer, and dementia, the FDA is addressing what Commissioner Marty Makary referred to as the "fear machine" that has dissuaded women from pursuing helpful treatments.
The updated labels now include age-specific guidance, particularly for women under 60 or within 10 years of menopause onset. This helps patients understand that their risks may differ significantly from older populations studied in earlier research. With clearer labeling, patients can make more informed decisions and explore a wider range of treatment options.
Expanded Access to Generic Options
In addition to the labeling updates, the FDA has approved the first generic version of Premarin (conjugated estrogens) in more than 30 years, addressing affordability concerns. This move is part of a broader effort to make hormone therapy more accessible. By offering a lower-cost alternative, this approval could help women who previously found brand-name treatments too expensive. FDA Commissioner Makary estimates that restrictive labeling once denied therapy to as many as 50 million women. With the combination of clearer labels and affordable options, the gap between those experiencing menopausal symptoms and those receiving treatment is expected to shrink significantly.
Conclusion
The FDA's November 2025 updates mark a significant shift in hormonal medication labeling, removing outdated black box warnings and rigid dosing restrictions. These long-standing barriers have often hindered effective and individualized care. By addressing the fact that earlier warnings were based on data from older populations and didn’t accurately reflect risks for younger women, the changes bring a more nuanced and relevant approach to treatment.
For patients, this means care that’s better tailored to their unique needs and risk profiles. The updated, age-specific guidance empowers women to make informed decisions about their treatment. Additionally, the clarification that local vaginal estrogen products don’t carry systemic risks eliminates yet another obstacle for those seeking relief from genitourinary symptoms.
These updates not only streamline treatment guidelines but also foster improved communication between patients and providers. As pharmaceutical companies roll out the new labeling in 2026, it’s important for patients to discuss these changes with their healthcare providers. This dialogue can help determine whether hormone therapy aligns with their health goals. The recent approval of the first generic Premarin in over three decades further enhances accessibility and affordability, creating new opportunities for women to explore treatment options that were previously out of reach.
Telehealth platforms like Oana Health are stepping in to bridge gaps in care. These services offer convenient access to licensed medical professionals who are well-versed in the latest FDA guidelines. With personalized treatment plans and medications delivered right to your doorstep, telehealth makes it easier than ever to navigate new hormonal therapy options. Whether you’re addressing hormonal imbalances, managing PCOS, or tackling weight concerns, these platforms provide evidence-based care tailored to the evolving landscape of women’s health.
FAQs
What do the new FDA labeling updates mean for the safety of hormone therapy in women under 60?
The FDA has revised its labeling for hormone therapy, eliminating black-box warnings related to cardiovascular disease, breast cancer, and probable dementia. However, the warning about the risk of endometrial cancer remains in place for estrogen-only products. These updates suggest a more balanced benefit-risk profile for women under 60, while still stressing the need to monitor specific health risks.
For women exploring hormone therapy, these changes underline the importance of consulting with a licensed healthcare provider. Tailored medical advice helps ensure that treatments align with individual health needs and address any potential risks effectively.
What does the removal of black box warnings for cardiovascular and breast cancer risks mean for hormonal medications?
The FDA’s decision to remove black box warnings for cardiovascular disease and breast cancer on hormonal medications lowers the most serious safety caution associated with these treatments. This shift could lead to more widespread prescribing and reshape how the risks of hormone therapy are viewed.
For patients, this change might open the door to more hormone therapy options. However, it’s crucial to have a thorough discussion with a licensed healthcare provider to weigh the potential risks and benefits and decide if these treatments align with your health needs.
What does the approval of generic Premarin mean for hormone therapy access?
The FDA has approved the first generic version of Premarin, offering a more budget-friendly option for those in need of estrogen-based hormone therapy. This development not only makes treatment more affordable but also improves access for individuals seeking care.
With this generic alternative now available, patients dealing with menopause symptoms or hormonal imbalances have greater flexibility in their treatment choices. This step ensures that more people can receive effective care without the financial burden often associated with hormone therapy.
